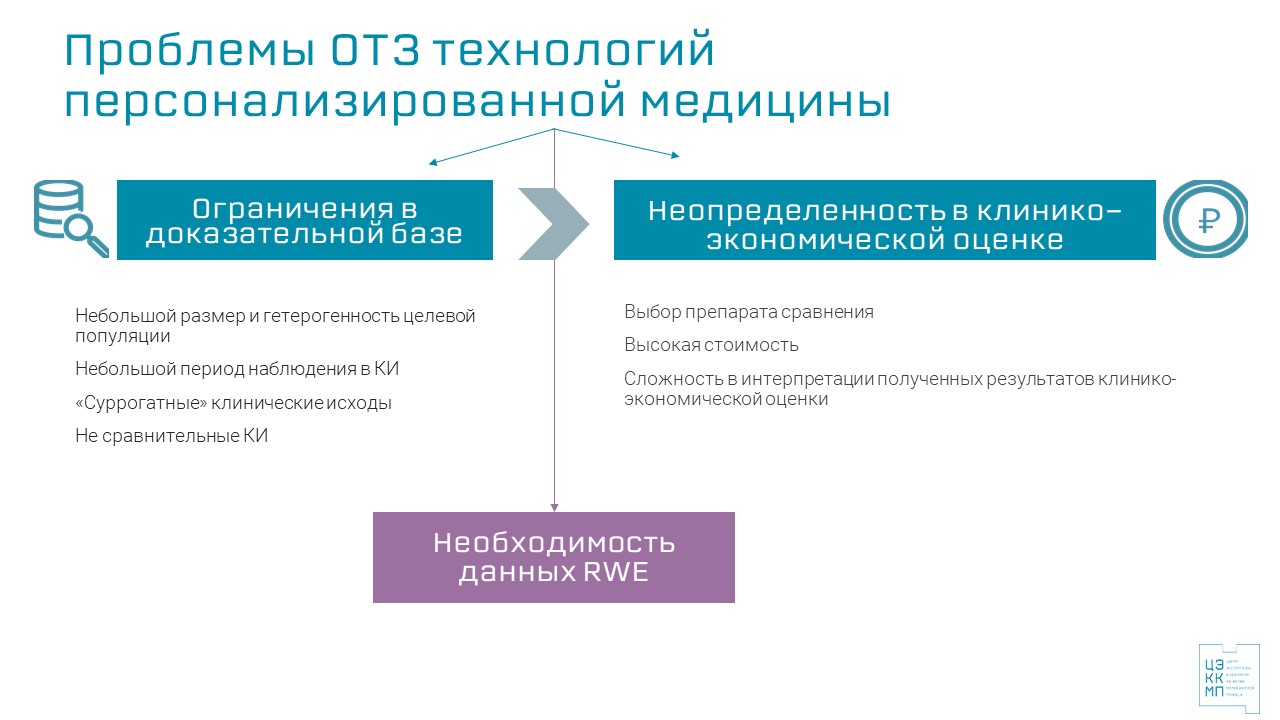
The extremely high cost of treatment, the limited evidence base for efficacy, especially in the long term, lead to a high degree of uncertainty in the clinical and economic assessment required for making a decision on the financing of such technologies.
In foreign countries, innovative models of drug provision (IMLO) - risk-sharing agreements - are widely used to include personalized technologies in the public funding system, which are based on data from real-world data. Existing IMLO models are classified as financial and outcome-based. Financial schemes are most often used due to the ease of administration. These include:
The use of outcome-based IMLO regimens requires administration costs, but these costs are justified in the case of extremely high cost of the drug and high uncertainty about the effect. Most often, such regimens are used for orphan drugs, gene and cell therapy, as they can increase cost efficiency and pay for the result. Nuria Musina, Advisor to the Head of the Federal State Budgetary Institution "CEKKMP" of the Ministry of Health of Russia: “The development of drugs towards ever greater personalization, the arrival of innovative personalized technologies raise the question of the availability of such technologies for patients. Their inclusion in the public funding system requires new approaches. The future of healthcare is the transition to value-based models of drug reimbursement and pricing (pay-per-result). " Source: https://rosmedex.ru/imlo/ |
||