Журнал «Реальная клиническая практика: данные и доказательства» нацелен на публикацию оригинальных исследований и обзоров, касающихся использования данных, полученных в рутинной медицинской практике, для оценки исходов лечения и принятия решений в области здравоохранения.
Опубликованные в журнале статьи охватывают, но не ограничиваются, такими ключевыми областями научных исследований, как использование регистров больных и нозологий, медицинских баз данных, электронных медицинских карт, потребление лекарственных препаратов, изучение исходов лечения в рутинной медицинской практике, анализ назначений в стационарных и амбулаторных условиях, безопасность применения лекарств, приверженность лечению, исследования сравнительной эффективности, клинико-экономический анализ, включая анализ стоимости болезни и бремя заболеваний, прагматические клинические исследования и большие упрощённые рандомизированные исследования, изучение методологии исследований на основе данных реальной клинической практики, включая сбор, отслеживание, поиск, совместное использование, анализ и интерпретацию «больших данных».
Также, на сайте журнала http://myrwd.ru/ публикуются новости, посвящённые проведённым исследованиям реальной клинической практики, конференциям, конгрессам и другим мероприятиям.
Журнал включён в перечень ВАК, рецензируемых научных изданий, в которых должны быть опубликованы основные научные результаты диссертаций на соискание ученой степени кандидата наук, на соискание ученой степени доктора наук. Научные специальности и соответствующие им отрасли науки, по которым издание включено в Перечень ВАК: 3.3.6. Фармакология, клиническая фармакология.
Журнал включён в Единый государственный перечень научных изданий (ЕГПНИ) — «Белый список» уровень 2 — перечень научных периодических изданий, созданный в целях оценки публикационной активности при публикации основных научных результатов диссертаций на соискание ученых степеней и/или результатов научно-исследовательских работ, учитываемых при оценке результативности деятельности научных, образовательных организаций высшего образования, научных сотрудников и профессорско-преподавательского состава.
Журнал зарегистрирован в Роскомнадзоре 23.11.2021 г., номер свидетельства ЭЛ № ФС 77 - 82354.
Текущий выпуск
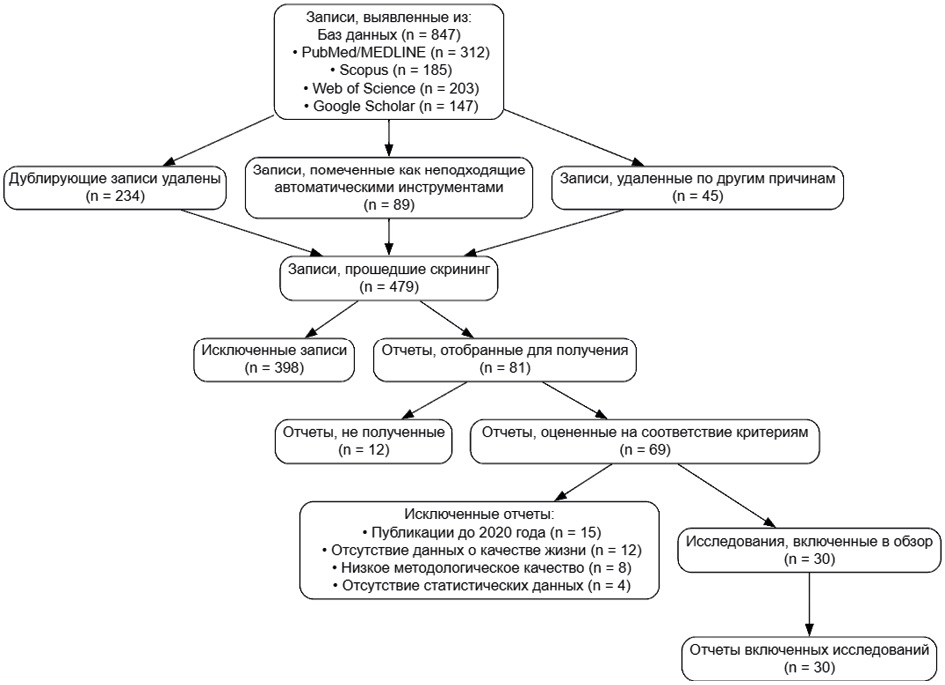
АКТУАЛЬНЫЕ ОБЗОРЫ
БЕЗОПАСНОСТЬ ЛЕКАРСТВ
КОГОРТНЫЕ ИССЛЕДОВАНИЯ
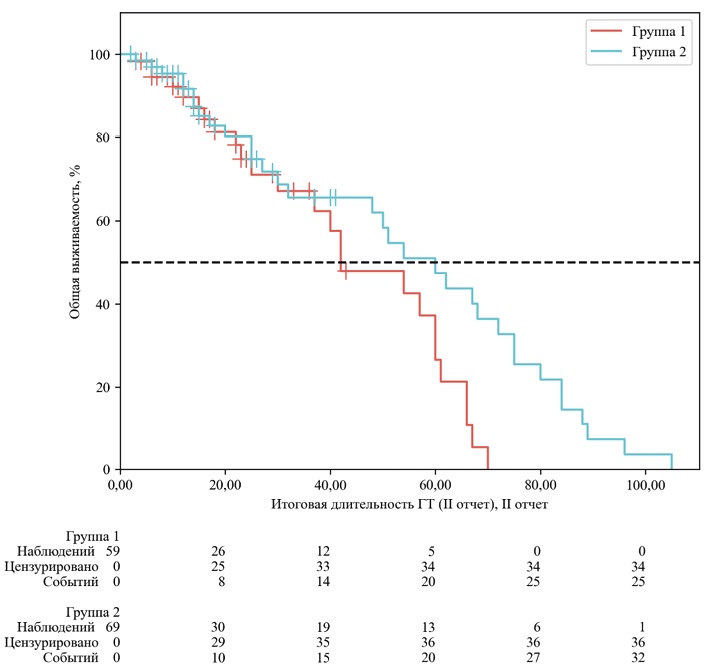
КАЧЕСТВО ЖИЗНИ
РЕЗОЛЮЦИЯ
Новости
2025-12-27
Качество данных в разработке лекарств: недостающий фундамент для реализации потенциала ИИ в клинических испытаниях
 | Трансформационный потенциал искусственного интеллекта (ИИ) в клинических исследованиях упирается в фундаментальную и часто игнорируемую проблему — качество и гармонизацию данных. В эпоху персонализированной медицины и взрывного роста объёмов информации (от носимых устройств до электронных медицинских карт) фрагментированные экосистемы данных становятся основным операционным барьером. |
2025-12-27
Использование реальных данных в регулировании медицинских изделий: новый этап с выпуском руководства FDA 2025 года
 | В декабре 2025 года FDA выпустило финальное обновленное руководство «Use of Real-World Evidence to Support Regulatory Decision-Making for Medical Devices». Этот документ знаменует собой новый этап в интеграции данных реальной клинической практики в процессы одобрения и мониторинга медицинских изделий. Статья анализирует ключевые положения руководства, его отличия от предыдущей версии 2017 года и практические последствия для производителей и исследователей. |
2025-12-24
Руководство FDA по использованию данных RWE для поддержки принятия регуляторных решений в отношении медицинских изделий
 | FDA завершило разработку руководства по использованию данных из реальной клинической практики (RWE) для поддержки принятия регуляторных решений в отношении медицинских изделий, заменив свою структуру 2017 года и завершив проект руководства 2023 года. |
2025-12-21
Школа RWD / RWE
 | Уже третий раз на базе кафедры клинической фармакологии и доказательной медицины "Первый Санкт-Петербургский государственный медицинский университет им. акад. И.П. Павлова" была проведена Образовательная программа дополнительного профессионального образования «Реальная клиническая практика: данные и доказательства» (Школа RWD / RWE). |
2025-12-19
FDA убирает ключевое препятствие для использования реальных данных
 | FDA официально разрешило использовать обезличенные данные пациентов из реальной клинической практики при подаче заявок на регистрацию медицинских изделий (а в перспективе — и лекарств). Это историческое изменение политики, принятое 15 декабря 2025 года. |
| Еще новости... |