2026-01-19
II Всероссийская конференция «Клиническая фармакология в педиатрии», 2026 г.
 | В рамках ХХVII Конгресса педиатров России с международным участием «Актуальные проблемы педиатрии» 14 февраля 2026 г. будет проведена II Всероссийская конференция «Клиническая фармакология в педиатрии». |
2026-01-19
Руководство ВОЗ по совершенствованию клинических исследований
 | В 2024 году Всемирная организация здравоохранения (ВОЗ) выпустила масштабное руководство «Руководство по наилучшей практике для клинических испытаний». Документ отвечает на запрос Всемирной ассамблеи здравоохранения (резолюция WHA75.8, 2022) и представляет собой стратегическую дорожную карту по реформированию глобальной системы клинических исследований. |
2026-01-16
Обзор новостей в области RWD / RWE за декабрь 2025 г.
 | Публикуем новости в мире и в РФ в области данных реального мира (RWD) и фактических данных реального мира (RWE) за декабрь 2025 года. |
2026-01-13
Актуальные вопросы доклинических и клинических исследований лекарственных средств и клинических испытаний медицинских изделий
 | 23-24 апреля 2026 года в Санкт-Петербурге состоится XIII Всероссийская конференция с международным участием «Актуальные вопросы доклинических и клинических исследований лекарственных средств и клинических испытаний медицинских изделий». |
2025-12-27
Качество данных в разработке лекарств: недостающий фундамент для реализации потенциала ИИ в клинических испытаниях
 | Трансформационный потенциал искусственного интеллекта (ИИ) в клинических исследованиях упирается в фундаментальную и часто игнорируемую проблему — качество и гармонизацию данных. В эпоху персонализированной медицины и взрывного роста объёмов информации (от носимых устройств до электронных медицинских карт) фрагментированные экосистемы данных становятся основным операционным барьером. |
2025-12-27
Использование реальных данных в регулировании медицинских изделий: новый этап с выпуском руководства FDA 2025 года
 | В декабре 2025 года FDA выпустило финальное обновленное руководство «Use of Real-World Evidence to Support Regulatory Decision-Making for Medical Devices». Этот документ знаменует собой новый этап в интеграции данных реальной клинической практики в процессы одобрения и мониторинга медицинских изделий. Статья анализирует ключевые положения руководства, его отличия от предыдущей версии 2017 года и практические последствия для производителей и исследователей. |
2025-12-24
Руководство FDA по использованию данных RWE для поддержки принятия регуляторных решений в отношении медицинских изделий
 | FDA завершило разработку руководства по использованию данных из реальной клинической практики (RWE) для поддержки принятия регуляторных решений в отношении медицинских изделий, заменив свою структуру 2017 года и завершив проект руководства 2023 года. |
2025-12-21
Школа RWD / RWE
 | Уже третий раз на базе кафедры клинической фармакологии и доказательной медицины "Первый Санкт-Петербургский государственный медицинский университет им. акад. И.П. Павлова" была проведена Образовательная программа дополнительного профессионального образования «Реальная клиническая практика: данные и доказательства» (Школа RWD / RWE). |
2025-12-19
FDA убирает ключевое препятствие для использования реальных данных
 | FDA официально разрешило использовать обезличенные данные пациентов из реальной клинической практики при подаче заявок на регистрацию медицинских изделий (а в перспективе — и лекарств). Это историческое изменение политики, принятое 15 декабря 2025 года. |
2025-12-12
Рецензия на книгу «Руководство по этике научных исследований» / под общей ред. А.Л. Хохлова. — М.: Изд-во ОКИ, 2026. — 764 с.
 | Для научного сообщества, ориентированного на исследования реальной клинической практики (Real-World Data/Evidence; RWD/RWE), выход фундаментального «Руководства по этике научных исследований» под редакцией академика РАН А.Л. Хохлова — событие не просто значимое, а стратегически важное. |
2025-12-11
Обзор новостей в области RWD / RWE за ноябрь 2025 г.
 | Публикуем новости в мире и в РФ в области данных реального мира (RWD) и фактических данных реального мира (RWE) за ноябрь 2025 года. |
2025-12-08
18–19 ноября 2025 г. в Сингапуре состоялось очное заседание Ассамблеи Международного совета по гармонизации (ICH)
 | В рамках которой параллельно проходили совещания 12 рабочих групп. В качестве новых членов ICH в заседании приняли участие Управление по контролю качества пищевых продуктов и лекарственных средств Нигерии (NAFDAC) и Южноафриканский орган по регулированию товаров медицинского назначения (SAHPRA), а в качестве новых наблюдателей – Главное управление по контролю качества лекарственных средств, пищевых продуктов и товаров медицинского назначения Доминиканской Республики (DIGEMAPS) и Управление по контролю качества пищевых продуктов и лекарственных средств (FDA) Филиппин. |
2025-12-05
На сайте ВОЗ опубликован новый документ «Глобальная стратегия интеллектуального фармаконадзора»
 | В ноябре 2025 года ВОЗ опубликовала документ «Глобальная стратегия интеллектуального фармаконадзора» (The global smart pharmacovigilance strategy). Стратегия ВОЗ задает рамки для построения и усиления национальных систем мониторинга безопасности с учетом разных уровней развития здравоохранения. |
2025-11-27
Наследственные патологии: как сделать их поиск частью повседневной практики педиатра
 | Наследственные заболевания могут проявляться с первых дней жизни ребенка, начиная от малых отклонений развития до угрожающих нарушений в работе сердца и дыхательной системы. Об этих симптомах, позволяющих диагностировать редкие и тяжелые заболевания, рассказали эксперты НИИ педиатрии и охраны здоровья детей НКЦ №2 ФГБНУ «РНЦХ им. акад. Б. В. Петровского» в ходе онлайн-конференции «Редкие заболевания в педиатрии: от симптома к системе» 18-19 ноября 2025 г. Организатором мероприятия выступил проект «Помощь редким». |
2025-11-26
Пилотная программа оценки комплексной ценности медицинского страхования на основе реальной клинической практики
 | Недавно Канцелярия Государственного управления медицинского страхования КНР выпустила «Уведомление о запуске пилотной программы оценки комплексной ценности медицинского страхования на основе реальной клинической практики» (№ 15, 2025), в котором принято решение о запуске пилотной программы в 11 провинциях и городах, включая Пекин и провинцию Хайнань. Эта пилотная инициатива знаменует собой выход управления медицинским страхованием Китая на новый этап: переход от решения задачи «обеспечения доступности» к решению задачи «повышения качества», используя данные реальной клинической практики для создания «точного профиля» фармацевтической продукции. |
2025-11-19
Российский биоаналог пембролизумаба показал эффективность в реальной клинической практике на Кубе
 | Российский биоаналог пембролизумаба (BCD-201), разработанный биотехнологической компанией BIOCAD, показал благоприятный профиль безопасности и эффективности в реальной клинической практике у пациентов с метастатической и неоперабельной меланомой. Данные получены в ходе наблюдательного исследования, которое проводилось в трёх крупнейших онкологических центрах Кубы с января по июль 2024 года. Данные представлены на международном онкологическом конгрессе ESMO в Берлине 20 октября 2025 года. |
2025-11-12
Обзор новостей в области RWD / RWE за октябрь 2025 г.
 | Публикуем новости в мире и в РФ в области данных реального мира (RWD) и фактических данных реального мира (RWE) за октябрь 2025 года. |
2025-11-12
Персональные данные в RWE-исследованиях: грядут большие перемены?
 | RWE-исследования, особенно основанные на вторичном использовании данных медицинских карт и регистров, упираются в серьезный барьер — техническую сложность получения согласия граждан на обработку их персональных данных и правовую неопределенность самого процесса обработки. |
2025-11-12
FDA использовала данные реальной клинической практики для подтверждения эффективности по препарату Vijoice® (алпелисиб / alpelisib)
 | Vijoice был одобрен FDA в апреле 2022 года для лечения пациентов с тяжёлыми формами синдрома избыточного роста, связанными с мутацией PIK3CA (PROS). |
2025-11-09
Проект руководства под названием «Методы сбора данных о безопасности и эффективности продуктов клеточной и генной терапии на пострегистрационном этапе»
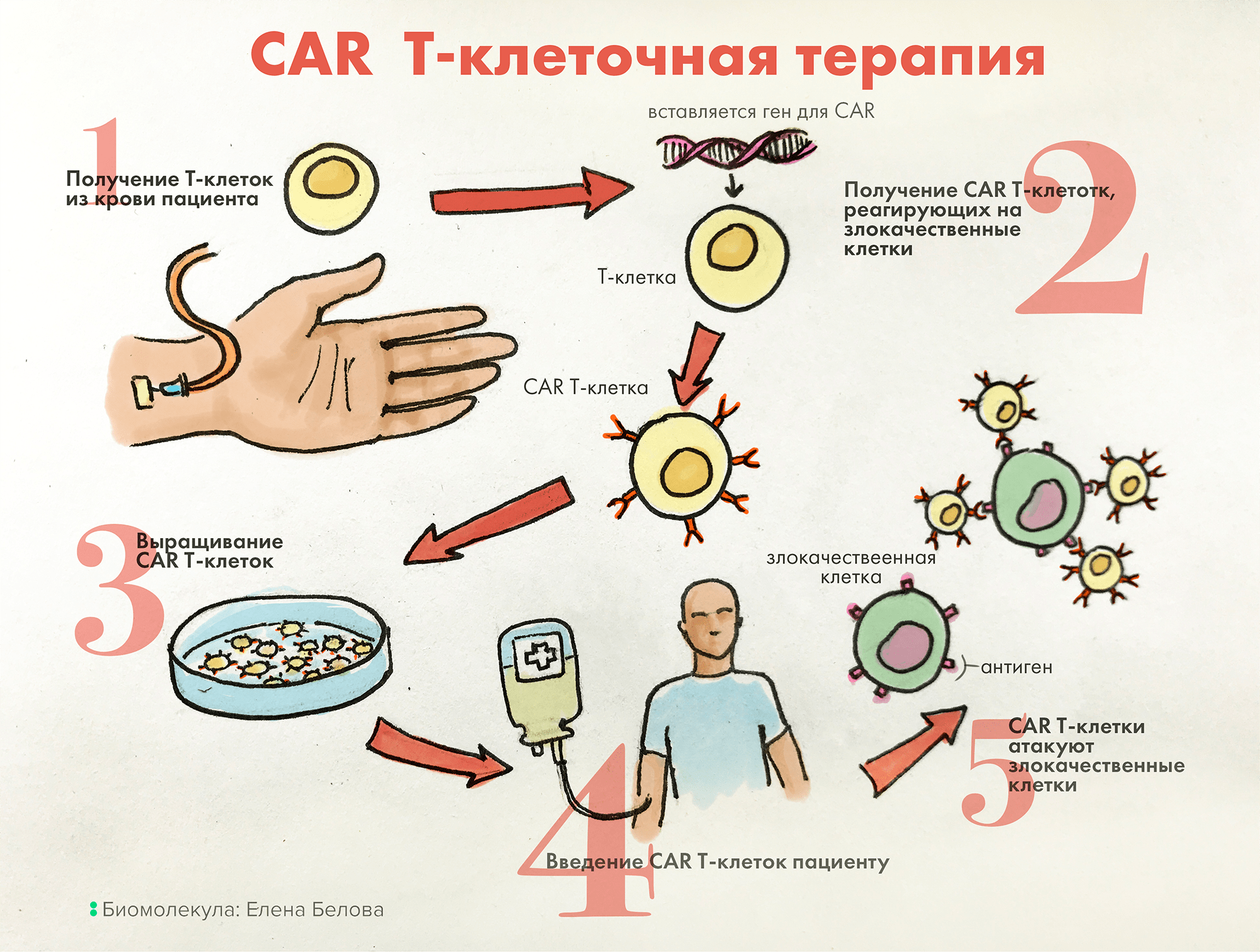 | FDA опубликовало проект руководства под названием «Методы сбора данных о безопасности и эффективности продуктов клеточной и генной терапии на пострегистрационном этапе», в котором разъясняется, как спонсорам следует собирать пострегистрационные данные, используя RWD, методы сбора данных за пределами традиционных клинических испытаний. |
2025-10-30
Европейская комиссия приняла окончательный вариант правила совместной клинической оценки (JCA) медицинских изделий и медицинских изделий для диагностики in vitro
 | Эта мера знаменует собой завершение формирования нормативной базы, необходимой для обеспечения единообразной и основанной на фактических данных оценки медицинских технологий на всей территории ЕС. |
2025-10-28
Интервью со спикерами VI конференции с международным участием: «Реальная клиническая практика. Возможное и реальное», Москва, 25 сентября 2025 года
2025-10-28
«Редкие заболевания в педиатрии: от симптома к системе»: 18-19 ноября 2025 эксперты обсудят внедрение принципов орфанного поиска в работу детских врачей
 | Редкие заболевания были и остаются вызовом, требующим от педиатра развитого клинического мышления и большой медицинской эрудиции. Тем не менее, современные представления о редких болезнях уже позволяют перейти от рассмотрения отдельных симптомов к системному взгляду на проблему — и органично внедрить принципы орфанного поиска в повседневную работу детского врача. |
2025-10-24
Развитие рынка медицинских данных в России требует особой модели регулирования
 | Ассоциация больших данных (АБД) разработала функциональную модель оценки риска при обмене чувствительными данными, включая медицинские данные, и занимается формированием стандартов в области риск-ориентированного подхода, рассчитывая на реализацию в России пилотных проектов по внедрению таких подходов. Об этом на сессии «Кибербезопасность в эпоху больших медицинских данных» форума «Национальное здравоохранение – 2025» сообщила директор по стратегическим проектам АБД Ирина Левова. |





























