Scroll to:
Paracetamol safety in COVID-19
https://doi.org/10.37489/2782-3784-myrwd-2
EDN: EZCLAJ
Abstract
The relevance of paracetamol safety assessment is associated with broad recommendations for its use as a symptomatic agent in COVID-19 and an agent for adverse events following vaccination to prevent COVID-19.
Objectives. Conducting a review of global paracetamol safety data during the initial period of COVID-19 pandemic (in 2020 and in the first half of 2021).
Materials and methods. 2,356 scientific articles and their abstracts on paracetamol safety in electronic libraries; data on 2,272 clinical trials of paracetamol in ClinicalTrials.gov and the State Register of Medicines of the Ministry of Health of the Russian Federation; 173,707 individual reports of adverse drugs reactions of paracetamol in the international pharmacovigilance database VigiBase. The data were evaluated by statistical methods in the VigiLyze analytical system (authorized expert access).
Results. No publications and completed clinical trials were found on the safety issue of paracetamol use in COVID-19. During the initial period of COVID-19 pandemic (from January 1, 2020 to July 31, 2021), the number of reports of paracetamol safety issues included in VigiBase decreased by 22,1%, which may be due to a decrease in the number of specialists sending reports on safety, with an increase of their duties and with a switching attention of these specialists and patients to other problems associated with the COVID-19 pandemic. No changes have been identified in the sources of safety data and trade names for paracetamol medicinal products. It has been established that the main part (99,4% of cases) of side effects associated with paracetamol develops outside the use of vaccines for the prevention of COVID-19. Pre-COVID and COVID data include information on 2,692 and 2,527 names of various paracetamol safety concerns, respectively. These data overlap the list of side effects specified in instructions for medical use and include new symptoms not described in instructions for medical use.
Conclusions. The lack of safety signals may be due to the lack of alertness of specialists and marketing authorization holders regarding paracetamol. During the COVID period, the structure and frequency of paracetamol safety concerns codified in terms of MedDRA (version 24.0) have changed, which can be studied in future studies of paracetamol, ibuprofen and other medicinal products.
Keywords
For citations:
Romanov B.K. Paracetamol safety in COVID-19. Real-World Data & Evidence. 2021;1(1):5-9. https://doi.org/10.37489/2782-3784-myrwd-2. EDN: EZCLAJ
Introduction
Paracetamol (the adopted international name – acetaminophen) was synthesized in 1877 by Harmon Northrop Morse, a chemist of Johns Hopkins University. The first clinical study of paracetamol was conducted in 1887 by the clinical pharmacologist Joseph von Mering. Paracetamol hematotoxicity (methemoglobinemia) revealed by him led to the fact that the drug product was forgotten for 60 years. In 1947-1948, two teams of researchers from the United States of America found that paracetamol toxic effect could be due to contamination with 4-aminophenol. This led to the formation of paracetamol safety reputation and its subsequent wide and long-term use – in 2021, the Register of Medicines of the Ministry of Health of the Russian Federation contains 208 medicinal products containing paracetamol [1]. However, there has been a gradual accumulation of new data on the risks associated with paracetamol administration in registered doses and in overdoses. The side effects of paracetamol are not fully studied (as well as their frequency), but their description usually takes several pages in instructions for its medical use [1]. Until now, pharmacodynamics of paracetamol associated with inactivation of two or three isoforms of cyclooxygenases in the central nervous system has not been established [1]. In 2020-2021 increased interest in paracetamol and the occurrence of a new aspect of relevance assessment of its safety are associated with broad recommendations for its use as the safest symptomatic antipyretic agent for mild and moderate COVID-19 (including in pregnant women, women in labor and postpartum women) in doses up to 4 g per day [2] and as an agent for side effects after immunization, which is specified in the COVID-19 Vaccination Participant's Memo given before the first vaccine administration.
Objectives: conducting a review of global paracetamol safety data during the initial period of COVID-19 pandemic (in 2020 and in the first half of 2021). Global pharmacovigilance data has been assessed for paracetamol in 2020-2021 and their comparison with data for the same time period in 2018-2019.
Materials and methods
When conducting a non-interventional assessment of the global data on paracetamol pharmacovigilance, there were used scientific articles and their abstracts posted in the electronic libraries eLibrary [3] and PubMed [4], as well as information from the international database of clinical trials ClinicalTrials.gov [5], data from the State Register of Medicines of the Ministry of Health of the Russian Federation [1] and global data from the international pharmacovigilance data system VigiBase [6] of Uppsala Monitoring Center of the World Health Organization for 2018-2021. Evaluation of the data obtained was carried out by statistical methods using the analytical system VigiLyze specialized for the implementation of expert functions of pharmacovigilance at the national and international levels in authorized access [6].
Results and discussion
The use of paracetamol during the COVID-19 pandemic is not a routine event, since patients with this nosological entity and even patients with suspicion of this disease, as well as those vaccinated against this disease, are under additional monitoring or included in clinical safety trials. In such non-standard conditions for paracetamol, a combination of pre- and post-authorisation safety studies should be done. It is necessary to be careful regarding quantitative assessment of safety indicators and expect an increase in the incoming information of a higher quality and publications of this data than for the general clinical practice of using such medicinal products. However, a complete lack of systematic reviews on paracetamol safety concerns in COVID-19 was found.
The search query "paracetamol" and "safety" in the Russian bibliographic system eLibrary showed 121 publications, including 32 publications for 2018-2021, which confirms the relevance of paracetamol safety assessment problems. Moreover, only one [7] of these publications mentions COVID-19 along with the recommendation of paracetamol and ibuprofen use for this disease, which confirms the novelty of this problem and its insufficient knowledge by domestic researchers.
The search query "acetaminophen and safety" in the bibliographic system of the US National Library of Medicine (PubMed), which indexes more than 30 million biomedical documents, showed 2,235 results, including 631 publications for 2018-2020, including 19 publications related to COVID-19, which also confirms the urgency of an issue. However, only one publication [7] out of 19 refers directly to paracetamol, highlighting only its effect on redox processes in COVID-19, which also confirms the novelty of the issue and its insufficient knowledge abroad.
The database of clinical trials ClinicalTrials.gov as of August 6, 2021, contained information on more than 385 thousand clinical trials conducted in 219 countries [5]. The search showed 2,227 clinical trials of paracetamol in this database, including 21 trials in COVID-19, of which only one trial of paracetamol effectiveness (at a dose of 6 g per day) in cancer patients with COVID-19, which will be completed in the United States of America in May 2023. Thus, none of the clinical trials of paracetamol in 2020-2021 included an assessment of its safety in COVID-19. The database of approvals for clinical trials on the website of the State Register of Medicines of the Ministry of Health of the Russian Federation [1] contains 45 records for paracetamol, including 6 records for 2020- 2021, but none of these records included an assessment of paracetamol safety in COVID-19.
Thus, search results in the largest specialized databases indexing most of the biomedical publications and data on clinical trials indicate a lack of information on the status of paracetamol safety concern in COVID-19.
As of August 6, 2021, the VigiBase database of the World Health Organization's International Safety Monitoring Program [6] included more than 27 million individual case safety reports (ICSRs) for medicinal products, including 173,707 ICSRs of adverse drug reactions when using paracetamol (all variants of the search terms "acetaminophen" and "paracetamol").
When conducting a comparative assessment of the number and contents of these ICSRs for 2020 and the first half of 2021 (up to July 31, 2021, including that date), that is during the COVID-19 pandemic, 23,989 ICSRs were obtained (hereinafter referred to as COVID data). At the same time, for a period of the same duration from January 1, 2018 to July 31, 2019 (hereinafter referred to as pre-COVID data), 35,744 ICSRs were received by VigiBase. Thus, during the COVID-19 pandemic, the number of reports of paracetamol safety concerns decreased by 22.1% (with a background of opposite trend expectations). The decrease in the number of ICSRs included in VigiBase may be due to a decrease in the number of specialists sending reports on safety, with an increase of their duties and with a switching attention of these specialists and patients to other problems associated with the COVID-19 pandemic.
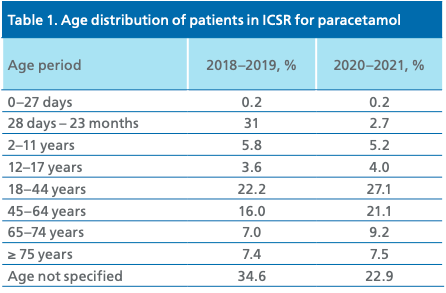
The age composition of patients with paracetamol safety concerns remained practically unchanged, while a quality reduction of ICSRs by one third (completeness) in this section can be noted – in COVID data, the number of ICSRs without age indicated increased by 33.8% (Table 1).
Also, the gender composition of patients with paracetamol safety concerns remained practically unchanged, while a quality increase of ICSRs (completeness) in this section can be noted – in COVID data, the number of ICSRs without sex indicated increased by 25.5% (Table 2).

The list of countries from which ICSRs were sent has hardly changed. This list generally corresponds to the list of countries that traditionally, for decades, send the maximum amount of safety data to VigiBase. TOP5 of these countries: USA, Republic of Korea, France, Malaysia and Singapore. All of these countries are in the ICH-zone of high regulatory requirements and have developed and effective pharmacovigilance systems, which may indicate the high value of the data obtained.
Also, the list of trade names of paracetamol medicinal products practically did not change. The largest number of ICSRs (approximately half of the total number) included information on generic paracetamol medicinal products that do not have their own trade names (43.7 and 57.0% in pre-COVID and COVID data, respectively). ICSRs for Tylenol (14.8 and 14.5%) are at the second place, ICSRs for Dolipran (8.3 and 3.6%) are at the third place.
Among ICSRs reporting the use of drugs other than paracetamol, the most common ICSRs with ibuprofen (0.7 and 0.6% in the pre-COVID and COVID data, respectively) and tramadol (0.4% in the pre-COVID and COVID periods). The use of paracetamol associated with the use of vaccine for the prevention of COVID-19 was noted in only 413 ICSRs (0.6% of COVID data). Thus, the main part of paracetamol side effects developed in unvaccinated patients (in 99.4% of cases).
Pre-COVID data in VigiBase include information on 2,692 names of various paracetamol safety concerns. These data completely overlap the list of side effects in instructions for medical use and include new symptoms not described in these instructions. At the same time, VigiBase does not generate safety signals on paracetamol, which may be due to the lack of alertness of specialists and marketing authorization holders regarding this drug.
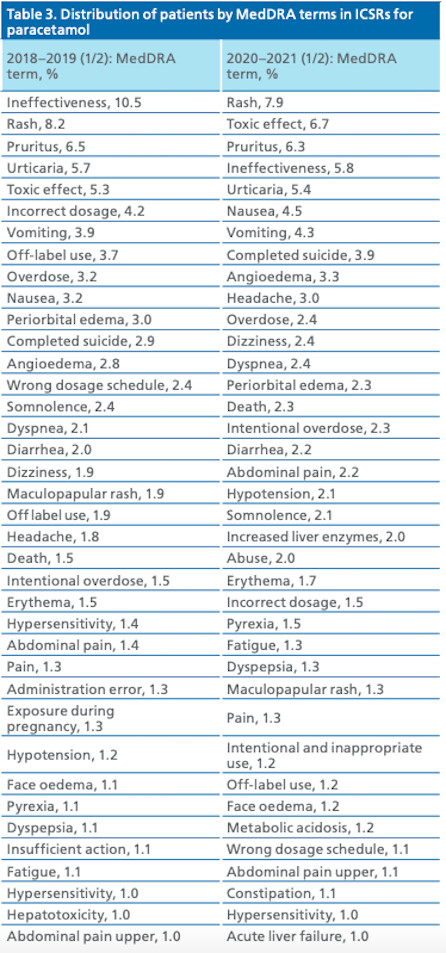
The most common (1% of ICSRs or more) terms of paracetamol safety concerns encoded according to the MedDRA regulatory classifier (version 24.0) are presented in Table 3. Noteworthy is the change in the structure of the list of the most common paracetamol safety concerns and the change in the frequency of their occurrence in ICSRs.
Sources of information on paracetamol safety concerns have not changed – most of the ICSRs were sent to VigiBase by doctors (32.6 and 32.5% in the pre-COVID and COVID periods, respectively) and pharmacists (21 and 21.9%).
Serious adverse reactions were more common in the COVID period (35.4 versus 28.2% in the pre-COVID period). In both periods, the leading criteria for severity were hospitalization or an increase in its duration (14.4 in the pre-COVID period and 17.6% in the COVID period), as well as other clinically significant events (12.3 and 16.3%). Abnormal development and congenital pathology were noted in 0.1 and 0.3%, respectively. The relative frequency of all deaths in cases of serious adverse events also increased in the COVID period – from 8.9 to 14.3%. It seems advisable to further research these findings, which can be represented by a dissertation topic or a series of dissertations that include situationally related drugs (paracetamol, ibuprofen, etc.).
Conclusions
1. There are no systematic reviews, articles and completed clinical trials on the safety issue of paracetamol use in COVID-19.
2. During the initial period of COVID-19 pandemic (from January 1, 2020 to July 31, 2021), the number of reports of paracetamol safety issues included in VigiBase decreased by 22.1%, which may be due to a decrease in the number of specialists sending reports on safety, with an increase of their duties and with a switching attention of these specialists and patients to other problems associated with the COVID-19 pandemic.
3. In the reported paracetamol safety concerns, the list of countries and trade names for paracetamol medicinal products have not changed.
4. The main part (99.4% of cases) of side effects associated with paracetamol develops outside the use of vaccines for the prevention of COVID-19.
5. Pre-COVID data include information on 2,692 names of various paracetamol safety concerns. These data overlap the list of side effects specified in instructions for medical use and include new symptoms not described in instructions for medical use. The lack of safety signals may be due to the lack of alertness of specialists and marketing authorization holders regarding paracetamol.
6. The COVID data include information on 2,527 names of various paracetamol safety concerns, which also completely overlap the list of side effects specified in instructions for medical use. These data also include new symptoms not described in instructions for medical use and also do not lead to the generation of safety signals.
7. During the COVID period, the structure and frequency of paracetamol safety concerns codified in terms of MedDRA (version 24.0) have changed, which can be studied in future studies.
References
1. State Register of Medicines of the Ministry of Health of the Russian Federation. [access date: August 6, 2021]. Accessed at: https://grls.rosminzdrav.ru/grls.aspx [Internet].
2. Temporary methodological recommendations “Prevention, diagnosis and treatment of new coronavirus infection (COVID-19). Version 11 (May 7, 2021)” approved by the Ministry of Health of the Russian Federation. 2021, 225 p. [access date: August 6, 2021]. Accessed at: https://clck.ru/UhXAg [Internet].
3. Elibrary. Scientific electronic library [access date: August 6, 2021]. Accessed at: https://elibrary.ru/defaultx.asp? [Internet].
4. PubMed. Scientific Electronic Library of the US National Library of Medicine [access date: August 6, 2021]. Accessed at: https://pubmed.ncbi.nlm.nih.gov [Internet].
5. ClinicalTrials.gov. Electronic Clinical Trial Database of the US National Library of Medicine [access date: August 6, 2021]. Accessed at: https://clinicaltrials.gov [Internet].
6. VigiLyze. Electronic database of the safety monitoring program [access date: August 6, 2021]. Accessed at: https://vigilyze.who-umc.org [Internet].
7. Leonova M.V. The use of nonsteroidal anti-inflammatory drugs and ibuprofen in COVID-19: a systematic review. Consilium Medicum. 2020; 22 (12): 31–36. DOI: 10.26442/20751753.2020.12.200558.
8. Verd S, Verd M. Paracetamol safety in COVID-19: Re: Antioxid Redox Signal 2021;10.1089/ars.2021.0017. Antioxid Redox Signal. 2021 Jul 23. doi: 10.1089/ars.2021.0128. Epub ahead of print. PMID: 34293954.
About the Author
B. K. RomanovRussian Federation
Boris K. Romanov – Dr. Sci. (Med.), Associate Professor. Head of Department of Pharmacology,
1 Ostrovityanova str., Moscow, 117997
Competing Interests:
The author declares no conflict of interest and no funding for this research work.
Review
For citations:
Romanov B.K. Paracetamol safety in COVID-19. Real-World Data & Evidence. 2021;1(1):5-9. https://doi.org/10.37489/2782-3784-myrwd-2. EDN: EZCLAJ






























