RESOLUTION
On September 29, 2022, the Association of Specialists in Health Technology Assessment, the Association of Clinical Pharmacologists, the St. Petersburg branch of the International Society for Pharmacoeconomic Research and Scientific Analysis (ISPOR) organized and held the III annual scientific and practical conference with international participation «Real clinical practice in a changing world. Challenges and Solutions». The topic of the conference brought together leading Russian and world experts in the field of RWD/ RWE.
METHODOLOGY
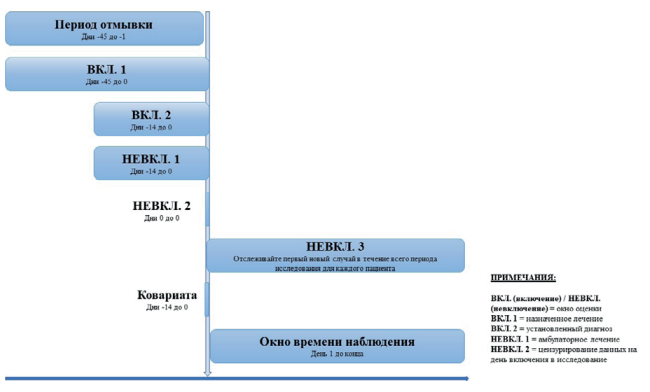
Aim. To highlight the features of STaRT-RWE (a structured template for planning and reporting on research in real clinical practice — RWE).
Materials and methods. One has selected and analyzed of the articles, dealing with the special features of getting evidence under conditions of real clinical routine, simplification of proving data collecting, in particular, at simultaneous consideration of several researches RWE.
Results. Due to the use of this pattern, one selects crucially important details. It is also important that the different interpretation does not influence the results and solvation.
Conclusion. STaRT-RWE is meant for decreasing the incorrect interpretation and making clear expectations as for generating RWE if to speak about the research design and its details, how and where these data should be revealed which will allow the persons, taking part in healthcare, estimate the RWE research reliability in an effective way.
INTERNATIONAL EXPERIENCE
The regulatory aspects of the conduction of the real-world clinical data studies became a trend in the past few years. In 2021 FDA issued three projects of such documents. The article resumes the main objectives of these FDA documents. Analysed projects of FDA recommendations represents the international expertise in this field and may be used in the process of building local regulations.
This article provides an overview of approaches to the use of real-world data (RWD) and real-world evidence (RWE) in the processes of registration and health technology assessment in Canada and the UK, as well as a brief description of tools for health management decision-making, the Canadian Real-world Evidence for Value in Cancer Collaboration and electronic databases and registries. Information is provided on when RWE submission is possible and recommended, and what requirements the regulatory agencies have for RWD and RWE.