HEALTH TECHNOLOGY ASSESSMENT
Assessment of the effectiveness of a drug is a complex process. Currently, the standard method for assessing the effectiveness of a medical intervention is the quality-adjusted life years (QALY). In the summer of 2024, a description of a new method for assessing the effectiveness of medical interventions — Standard of Living Valuation (SoLV) was published. The proposed method is more difficult than QALY, but its capabilities are broader. However, this method is also not optimal. The problem of creating an optimal method for assessing the cost-effectiveness of health technologies remains open.
Biosimilars face significant challenges in assessing their value. The rapid development of this area of t he pharmaceutical industry requires a rethinking of the economic evaluation of drugs and optimization of regulatory procedures.
DRUG SAFETY
Relevance. Anticoagulants are commonly used during pregnancy is a fairly common practice. Due to the widespread use of oral anticoagulants in real practice and the high frequency of unplanned pregnancy, physicians should assess the risks and plan pregnancy management strategies.
Objective. The purpose of this analysis was to assess the legal background and evidence on the risks associated with the use of oral anticoagulants in pregnant women in real-world clinical practice.
Materials and methods. An analysis of the official instructions for the use of oral anticoagulants registered in Russia, an analysis of the published literature on reproductive toxicity and the use of drugs during pregnancy, and a general comparative analysis of the predominance of individual oral anticoagulants in practical healthcare in Russia.
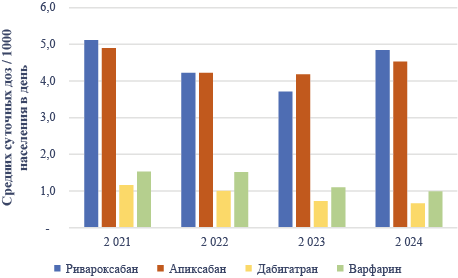
Results. The results of the analysis revealed high variability in the restrictions on the use of oral anticoagulants during pregnancy. At the same time, the actual evidence of reproductive toxicity for drugs does not differ significantly and indicates the absence of signals of potential teratogenicity, at least based on the available number of observations. The maximum number of pregnancy observations exceeding the number of observations on warfarin use was reported for the use of rivaroxaban (505 cases in the largest analysis). The remaining representatives are described by several dozen observations. In Russia, the use of oral anticoagulants is also a prerequisite for the more likely use of rivaroxaban and apixaban in cases of unplanned pregnancy.
Conclusions. In real-world clinical practice, exposure to oral anticoagulants is a prerequisite for pregnancy, which requires decision-making on strategies for pregnancy management. Instructions for the use of drugs do not contain reasonable information or formulations for decision-making based on accumulated knowledge about reproductive toxicity. Based on published data on the use of drugs in this group during pregnancy in real-world clinical practice, there is no sign of teratogenic potential for all representatives, with the exception of warfarin, whereas the largest number of observations concerns the most commonly used rivaroxaban.
DIGITAL HEALTHCARE TECHNOLOGIES
This article is devoted to the study and analysis of electronic health records (EHR), which are the main tool in the field of digital healthcare. EHR provide faster and more efficient transfer of medical information between various institutions and specialists, which contributes to improving the quality of medical care. The article discusses various aspects of the implementation and use of EHR, including technical difficulties, data privacy and security issues. Special attention is paid to the influence of EHR on the processes of diagnosis and treatment, as well as on the interaction between patients and medical professionals. Based on a review of current research and practical examples, the authors propose recommendations for optimizing the use of EHR in medical institutions. In conclusion, the prospects for further development of EHR technologies and their role in the transformation of modern medicine are discussed.
ORIGINAL RESEARCH
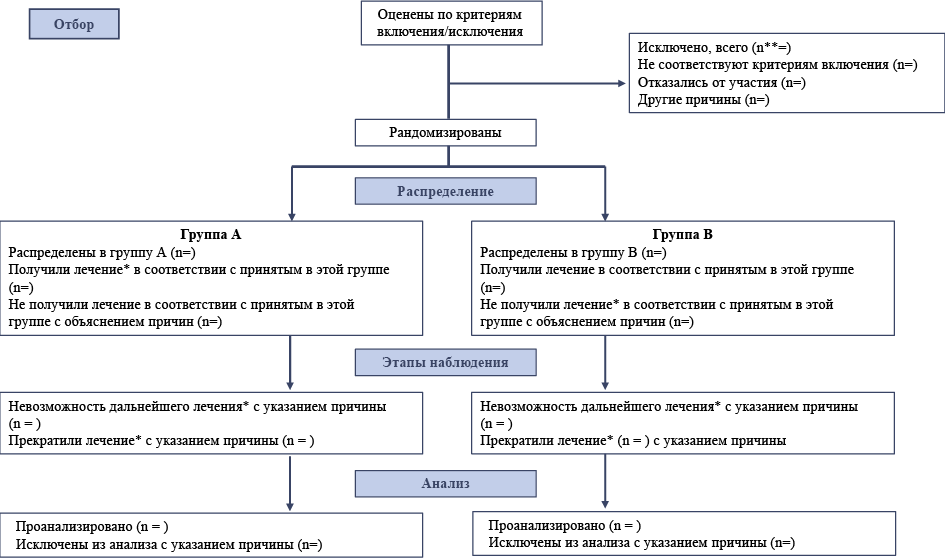
The gold standard for presenting evidence of the effectiveness and safety of medical procedures is randomized controlled trials (RCTs). For the research results to be available to other specialists and have potential weight in the scientific community, they must be described and published in accordance with generally accepted international standards, CONSORT, or Consolidated standards of reporting trials.
Objective: to analyze the quality of presenting the results of clinical trials in the Russian Federation in accordance with CONSORT standards.
Materials and methods. The following Russian journals were selected for the study: "Scientific and Practical Rheumatology", "Good Clinical Practice", "Social and Clinical Psychiatry", "S. S. Korsakov Journal of Neurology and Psychiatry". The selection of target journals was based on two main indicators of the journal rating in the Russian Science Citation Index; according to the SCIENCE INDEX rating and the rating of psychiatric journals by the impact factor RSCI 2016. Journals in the fields of biomedicine (Scientific and Practical Rheumatology), clinical pharmacology (Good Clinical Practice), and journals in the fields of psychology and psychiatry (Social and Clinical Psychiatry, S. S. Korsakov Journal of Neurology and Psychiatry) were selected. Each article was assessed according to the CONSORT checklist items, and the presence or absence of data on the presented items was noted. When studying the articles, the presence or absence of a flowchart in the article was also analyzed, which significantly improves the quality of the written article and compliance with international standards for the design of articles for medical journals.
Results. The articles analyzed with the results of RCTs in journals met most of the necessary criteria. The authors paid the least attention to such details of the methodology when publishing a description of the sample calculation (2 out of 5 publications), the type of randomization (2 out of 5 publications), the mechanism of blinding participants (3 out of 5 publications), and implementation and masking (1 out of 5 publications). The inaccessibility of this information may have distorted the reader’s perception and trust in the results.
Conclusion. The results show that the reviewed Russian medical journals declare requirements and recommendations for the publication of articles in accordance with the CONSORT standards. To increase readers' confidence in the practical and scientific significance of RCTs, it is necessary to consider the CONSORT requirements and strive to follow them.
EDUCATION
This article discusses the importance of pharmacoepidemiological studies in assessing the knowledge and preferences of specialists. This section outlines the author’s experience in organizing and conducting such studies, provides a rationale for the term "educational pharmacoepidemiology" (EPE), and presents information about the establishment of the first EPE laboratory. In the context of critical areas of evidence-based medicine, such as "Real-World Data" and "Real-World Evidence", the introduction of a bridging term, "Real-World Knowledge" (RWK), is justified.
BOOK REVIEW
The 2nd revised and supplemented edition of “Pharmacovigilance” has been published. This book discusses the drug safety and pharmacovigilance systems from the viewpoint of clinical pharmacology. The history of drug safety studies is given in details. The main definitions and classifications of adverse events are presented below. A separate chapter will examine the pharmacovigilance system in the EAEU member states. The textbook is intended for students, residents, postgraduates, clinical pharmacologists, physicians of other specialties, holders of registration certificates, and researchers.