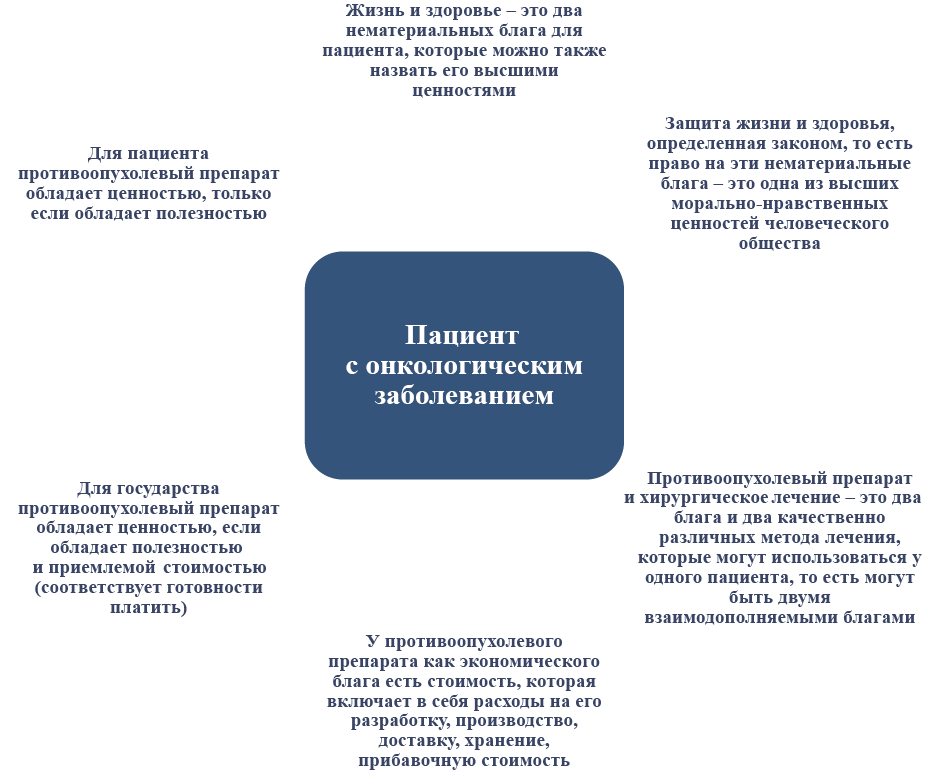
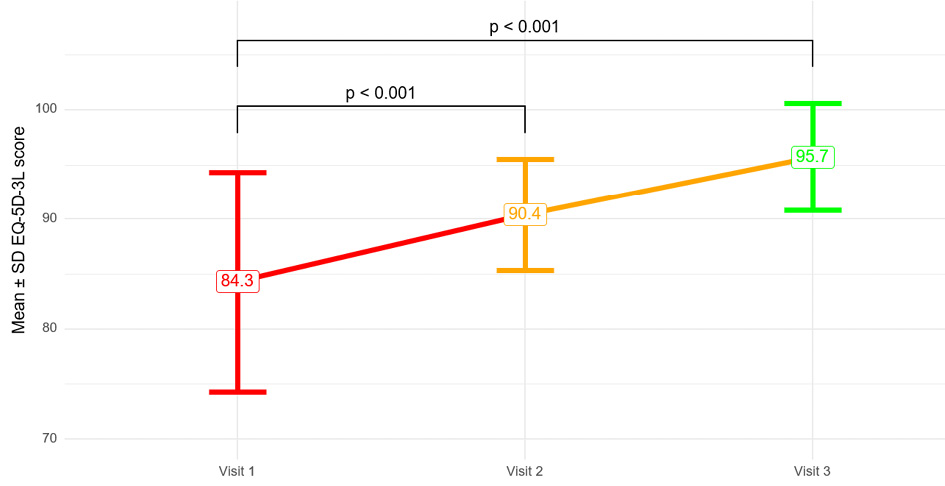
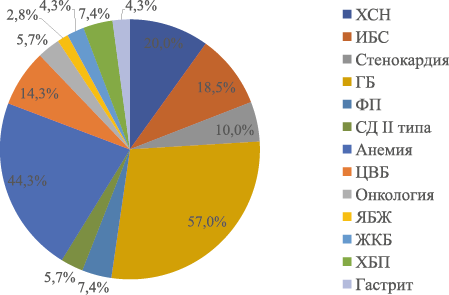
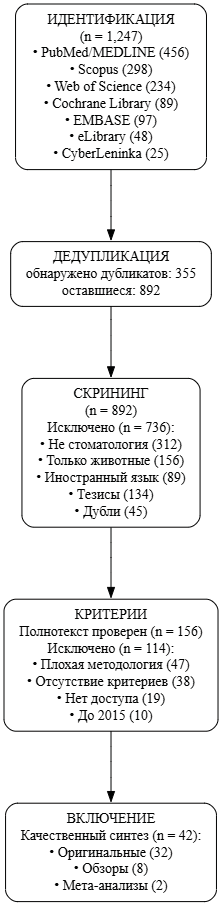
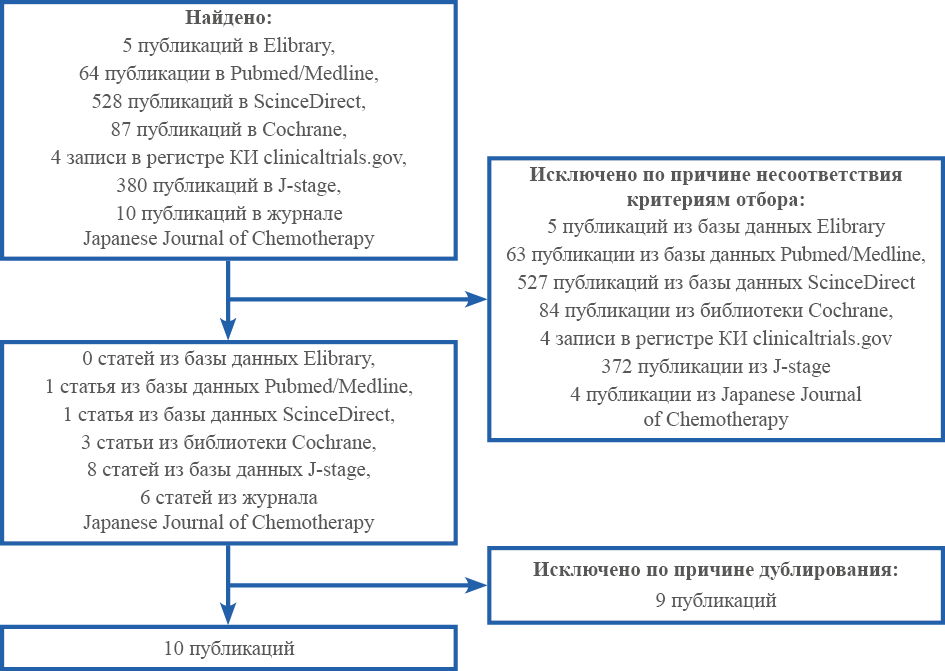
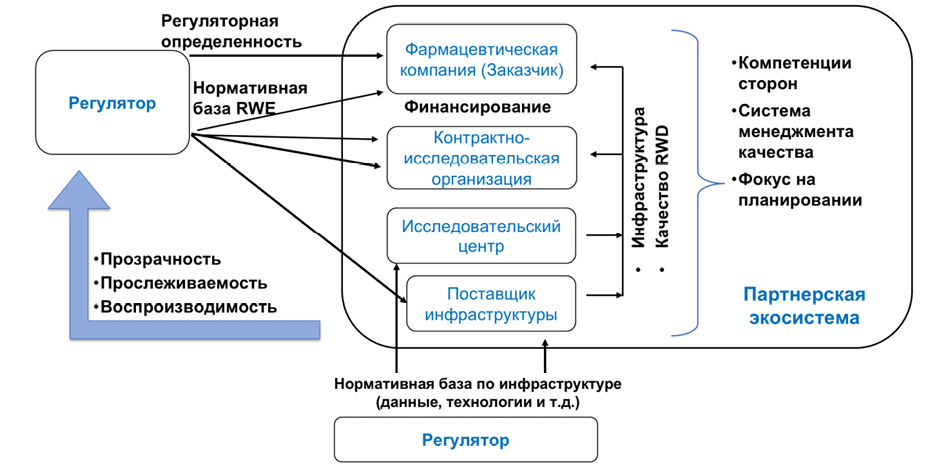
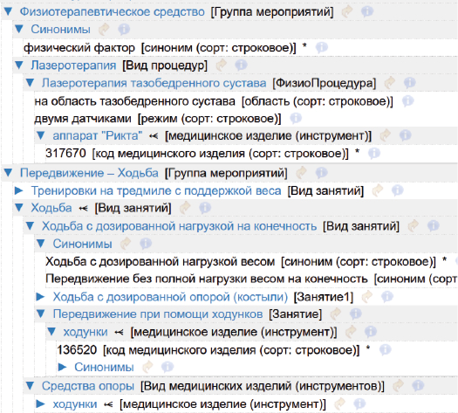
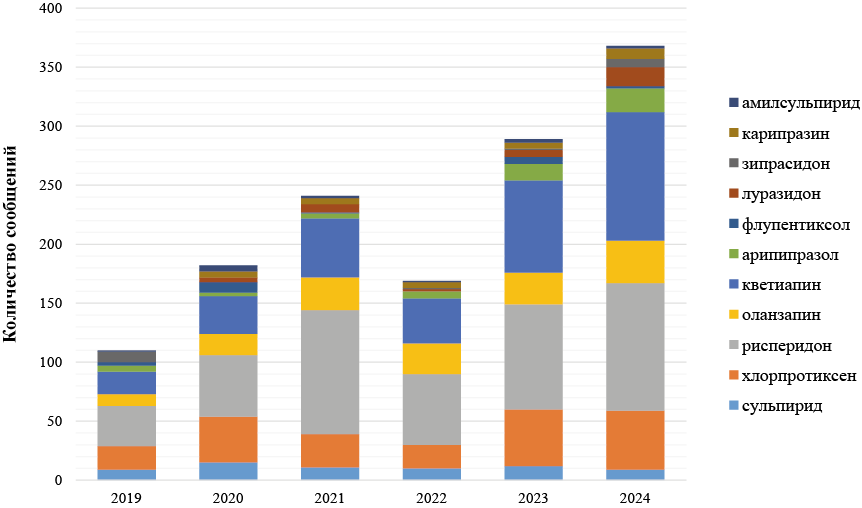Журнал «Реальная клиническая практика: данные и доказательства» нацелен на публикацию оригинальных исследований и обзоров, касающихся использования данных, полученных в рутинной медицинской практике, для оценки исходов лечения и принятия решений в области здравоохранения.
Опубликованные в журнале статьи охватывают, но не ограничиваются, такими ключевыми областями научных исследований, как использование регистров больных и нозологий, медицинских баз данных, электронных медицинских карт, потребление лекарственных препаратов, изучение исходов лечения в рутинной медицинской практике, анализ назначений в стационарных и амбулаторных условиях, безопасность применения лекарств, приверженность лечению, исследования сравнительной эффективности, клинико-экономический анализ, включая анализ стоимости болезни и бремя заболеваний, прагматические клинические исследования и большие упрощённые рандомизированные исследования, изучение методологии исследований на основе данных реальной клинической практики, включая сбор, отслеживание, поиск, совместное использование, анализ и интерпретацию «больших данных».
Также, на сайте журнала http://myrwd.ru/ публикуются новости, посвящённые проведённым исследованиям реальной клинической практики, конференциям, конгрессам и другим мероприятиям.
Журнал включён в перечень ВАК, рецензируемых научных изданий, в которых должны быть опубликованы основные научные результаты диссертаций на соискание ученой степени кандидата наук, на соискание ученой степени доктора наук. Научные специальности и соответствующие им отрасли науки, по которым издание включено в Перечень ВАК: 3.3.6. Фармакология, клиническая фармакология.
Журнал включён в Единый государственный перечень научных изданий (ЕГПНИ) — «Белый список» уровень 2 — перечень научных периодических изданий, созданный в целях оценки публикационной активности при публикации основных научных результатов диссертаций на соискание ученых степеней и/или результатов научно-исследовательских работ, учитываемых при оценке результативности деятельности научных, образовательных организаций высшего образования, научных сотрудников и профессорско-преподавательского состава.
Журнал зарегистрирован в Роскомнадзоре 23.11.2021 г., номер свидетельства ЭЛ № ФС 77 - 82354.
Текущий выпуск
ОЦЕНКА ТЕХНОЛОГИЙ ЗДРАВООХРАНЕНИЯ
НАБЛЮДАТЕЛЬНЫЕ ИССЛЕДОВАНИЯ
БЕЗОПАСНОСТЬ ЛЕКАРСТВ
СИСТЕМАТИЧЕСКИЙ ОБЗОР И МЕТААНАЛИЗ
МНЕНИЯ ЭКСПЕРТОВ
НОВЫЕ МЕДИЦИНСКИЕ ТЕХНОЛОГИИ
Новости
2026-01-26
Международная научно-техническая конференция «Микроэлектронные имплантируемые нейроинтерфейсы 2026» (МИН-2026)
 | 21-22 октября 2026 г. на площадке МИЭТ в гибридном формате будет проведена международная научно-техническая конференция «Микроэлектронные имплантируемые нейроинтерфейсы 2026» (МИН-2026). |
2026-01-23
28 - 30 мая 2026 г. в Казани состоится главное событие года по медицине боли в России - XXXII Всероссийский конгресс с международным участием «МЕДИЦИНА БОЛИ 2026»
 | Конгресс будет интересен неврологам, терапевтам, врачам общей практики, нейрохирургам, анестезиологам, травматологам, ревматологам, педиатрам, мануальным терапевтам, психиатрам, онкологам, урологам, гинекологам, реабилитологам, клиническим фармакологам, организаторам здравоохранения, а также другим специалистам, чья профессиональная деятельность связана с изучением, диагностикой и лечением боли. |
2026-01-19
II Всероссийская конференция «Клиническая фармакология в педиатрии», 2026 г.
 | В рамках ХХVII Конгресса педиатров России с международным участием «Актуальные проблемы педиатрии» 14 февраля 2026 г. будет проведена II Всероссийская конференция «Клиническая фармакология в педиатрии». |
2026-01-19
Руководство ВОЗ по совершенствованию клинических исследований
 | В 2024 году Всемирная организация здравоохранения (ВОЗ) выпустила масштабное руководство «Руководство по наилучшей практике для клинических испытаний». Документ отвечает на запрос Всемирной ассамблеи здравоохранения (резолюция WHA75.8, 2022) и представляет собой стратегическую дорожную карту по реформированию глобальной системы клинических исследований. |
2026-01-16
Обзор новостей в области RWD / RWE за декабрь 2025 г.
 | Публикуем новости в мире и в РФ в области данных реального мира (RWD) и фактических данных реального мира (RWE) за декабрь 2025 года. |
| Еще новости... |