RETRACTED ARTICLE
Introduction. CDK4/6 inhibitors in combination with endocrine therapy (ET) are the current standard of care for patients with HR+ HER2- advanced and metastatic breast cancer (eBC and mBC). Evidence of the clinical efficacy of CDK4/6 inhibitors has been obtained in several randomized clinical trials (RCTs), but data on their efficacy in real-world clinical practice (RCP) are contradictory.
Objective. The aim of the ICEDORA study was to analyze clinical and demographic characteristics, treatment regimens, and clinical outcomes in patients with HR+ HER2- mBC and mBC receiving CDK4/6 inhibitors in a real-world clinical practice in Moscow.
Materials and methods. ICEDORA is a non-interventional retrospective study based on the analysis of data on patients receiving CDK 4/6 inhibitors in Moscow. Clinical characteristics and treatment details of all patients with HR+ HER2-breast cancer who received ribociclib, palbociclib, or abemaciclib from January 2020 to the end of December 2022 were extracted from primary medical records (outpatient charts and medical histories) by parsing. Overall survival (OS) was calculated from the date of breast cancer diagnosis until patient death using the Kaplan-Meier method, and differences between treatment groups were assessed using the log-rank test. Multivariate logistic regression and Cox proportional hazards models were built to exclude the influence of confounders and systematic errors.
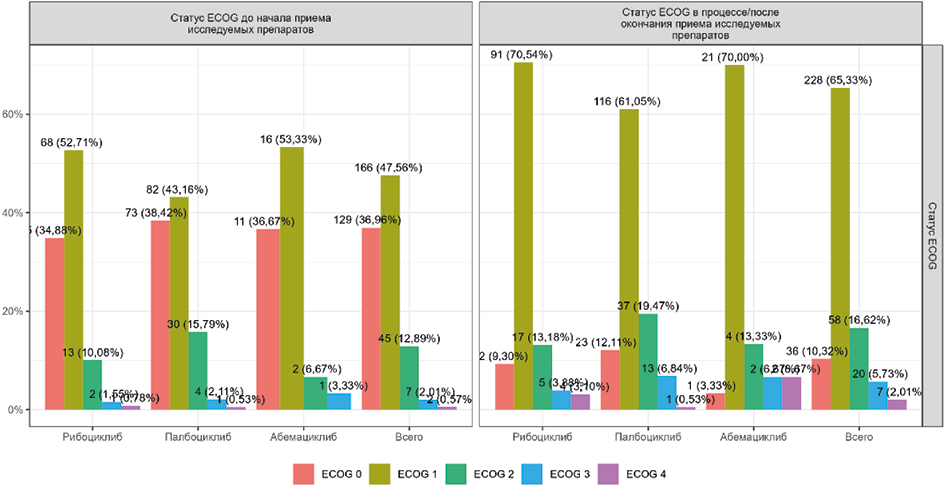
Results. The analysis included 2051 patients. 58.7 % received palbociclib, 34.7 % — ribociclib, 6.6 % — abemaciclib. The median age in the overall population was 58 years, but it was higher in the abemaciclib group (62 years). In 83.5 % of cases, Her2/neu was negative, the groups were homogeneous in this indicator. The level of estrogen receptors (ER) Ki-67 was significantly higher in the abemaciclib group. In the overall population, patients with primary metastatic breast cancer (stage IV) accounted for 42.1 %. The distribution of stages within each group was comparable. The groups differed significantly in the number of metastases due to a higher proportion of patients with one metastasis in the abemaciclib group (31.9 %) compared to the ribociclib (19.0 %) and palbociclib (16.6 %) groups, where the number of metastatic foci was more than one. Comorbidity was present in 90 % of patients. Differences in the baseline characteristics of patients receiving different drugs require caution when interpreting the study results.
Conclusion. The ICEDORA study, which included patients taking CDK4/6 inhibitors in real-world clinical practice in Moscow, is one of the largest analyses of clinical and demographic characteristics, treatment regimens, and clinical outcomes in patients with HR+ HER2- mBC and mBC taking CDK4/6 inhibitors in real-life clinical practice in Russia. Comparative analysis of the efficacy of ribociclib, palbociclib, and abemaciclib in real-life clinical practice was not planned or conducted due to differences in the clinical and demographic characteristics of patients. Larger multicenter data with
balanced cohorts and long-term follow-up are needed.